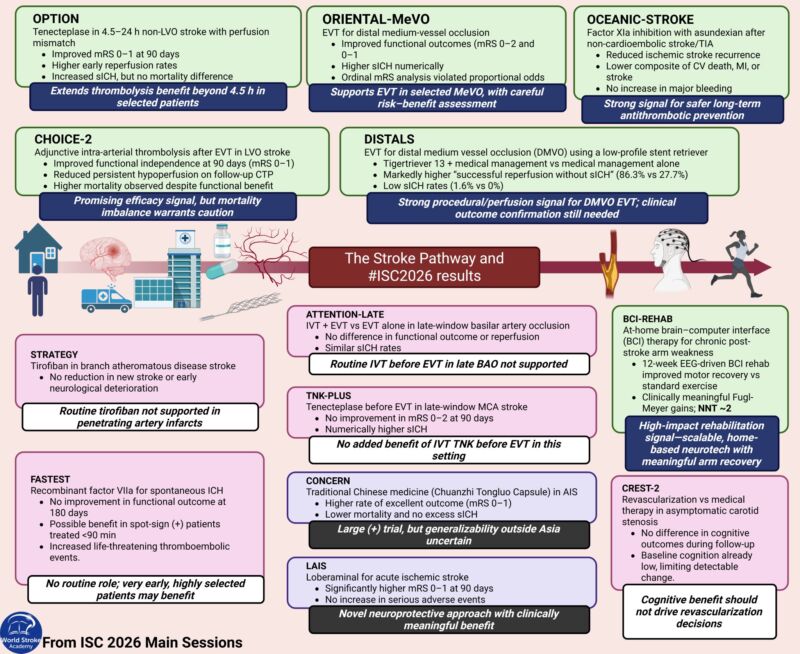
The Paper of The Month – May
21 May 2024Controversies in Stroke Recovery: is there a role for Fluoxetine?
Controversies in Stroke Recovery: is there a role for Fluoxetine?
By Prof. Dr. Gustavo Saposnik
“I have never entered into any controversy in defense of my philosophical opinions; I leave them to take their chance in the world. If they are right, truth and experience will support them; if wrong, they ought to be refuted and rejected…”
Benjamin Franklin (1706-1790) statesman, scientist, inventor and diplomat
Commentary
This month, we examine a publication that addresses a longstanding controversy: the role of Fluoxetine in stroke recovery.[1]
In 2011, the publication of the FLAME study sparked considerable interest among clinicians.[2] In this double-blind, placebo-controlled trial, 118 patients, aged 18 to 85 years, who experienced hemiplegia or hemiparesis with a Fugl-Meyer motor scale (FMMS) score of 55 or less, were randomly assigned to receive either fluoxetine (n=59) or placebo (n=59) within 5-10 days following an ischemic stroke.[2] All patients received physiotherapy.
It’s important to note the concept of the minimal clinically important difference (MCID), defined as the smallest difference in score within the domain of interest that patients perceive as beneficial.[3, 4] For these patients, the MCID for the FMMS is reported to be between 10 and 12 points.[5, 6]
The primary outcome assessed in the FLAME study was the change in FMMS score between day 0 and day 90 after treatment allocation. The authors observed a significant improvement in FMMS at day 90 in the fluoxetine group (adjusted mean 34·0 points [95% CI 29·7-38·4]) compared to the placebo group (24·3 points [19·9-28·7]; p=0·003).[2]
However, subsequent trials and meta-analyses[7, 8] have failed to replicate these findings, showing no significant benefits of Fluoxetine compared to placebo.
This interview highlights the most recent publication and the current state of the art. In summary, Fluoxetine does not appear to improve motor recovery after stroke and may potentially increase the risks of hyponatremia, bone fractures, and seizures.
References
- Mead, G., et al., Individual patient data meta-analysis of the effects of fluoxetine on functional outcomes after acute stroke. Int J Stroke, 2024: p. 17474930241242628.
- Chollet, F., et al., Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol, 2011. 10(2): p. 123-30.
- Hsieh, Y.-W., et al., Establishing the minimal clinically important difference of the Barthel Index in stroke patients. Neurorehabilitation and neural repair, 2007. 21(3): p. 233-238.
- Revicki, D., et al., Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. Journal of clinical epidemiology, 2008. 61(2): p. 102-109.
- Hiragami, S., Y. Inoue, and K. Harada, Minimal clinically important difference for the Fugl-Meyer assessment of the upper extremity in convalescent stroke patients with moderate to severe hemiparesis. J Phys Ther Sci, 2019. 31(11): p. 917-921.
- Narayan Arya, K., R. Verma, and R. Garg, Estimating the minimal clinically important difference of an upper extremity recovery measure in subacute stroke patients. Topics in stroke rehabilitation, 2011. 18(sup1): p. 599-610.
- Legg, L.A., et al., Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery. Cochrane Database Syst Rev, 2021. 11(11): p. Cd009286.
- Liu, G., et al., Is Fluoxetine Good for Subacute Stroke? A Meta-Analysis Evidenced From Randomized Controlled Trials. Front Neurol, 2021. 12: p. 633781.
Interview to Prof. Dr. Gillian Mead
What did you set out to study?
There was great excitement when the small French trial called FLAME (more than ten years ago now) found that fluoxetine given early after stroke appeared to improve motor recovery. This needed to be confirmed in large trials. And so a UK team led by myself and Martin Dennis joined forces with Sweden (Erik Lundström and the late Veronica Murray) and Australia (Graeme Hankey and Maree Hackett) to design three trials based on the same protocol, to implement in different countries.
Why is this important?
The results of these three large trials designed with the same protocol (EFFECTS, AFFINITY and FOCUS) showed that fluoxetine did not improve stroke recovery when given early after stroke. But it’s possible that each individual trials might have missed a small effect size, or couldn’t identify specific subgroups who might benefit or perhaps didn’t have enough outcome events to precisely estimate risks. That’s why we had decided to do an individual patient data meta-analysis.
What were the key findings?
Our key findings, in a dataset of more than 6000 patients, were that fluoxetine did not improve modified Rankin scale at 6 months (our primary outcome). It did reduce depression risk but increased fractures, falls with injuries, bone fractures and hyponatraemia. There were no interaction with the minimisation variables or in subgroups (including those with motor deficits at baseline).
How might these results impact clinical practice?
These results are important because we can now be certain that fluoxetine does not improve recovery when given routinely early after stroke.
It did surprise me a bit that there was no increased risk of bleeding, which had been a concern when we designed the protocol because of the multiple pharmacological actions of fluoxetine on clotting and platelet function.
What’s next?
We are planning further analyses using the IPD dataset to look in depth at the characteristics of people who suffer fracture and those who are at risk of seizures. We have data on other outcomes that are important to patients including fatigue, mood and pain, and so can develop predictive models to identify those most at risk.
I would like to thank all the participants and families, the patient and public advisors, our sites, our collaborators, co-authors of this paper, and of course our funders. The three trials were a massive team effort by many many people and it’s been a pleasure and privilege to be have been involved in leading such impactful research.