The Paper of The Month – June
15 Jun 2023Title: Managing Efficiency in Acute Stroke care
Title: Managing Efficiency in Acute Stroke care
Author: Dr. Gustavo Saposnik, EiC, WSA
This article is a commentary on the following: The third Intensive Care Bundle with Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT3): an international, stepped wedge cluster randomised controlled trial, https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(23)00806-1/fulltext
“Knowing is not enough; we must apply. Willing is not enough; we must do.”
Johann Wolfgang von Goethe (German Writer 1749-1832)
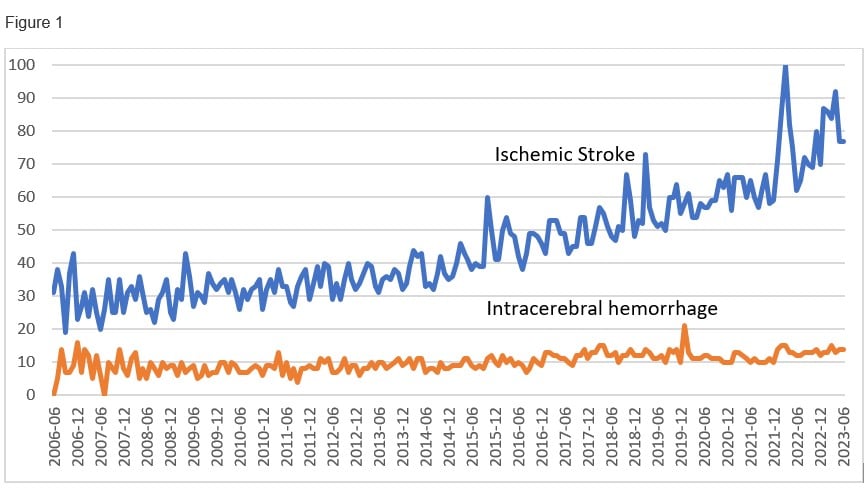
The management of intracerebral hemorrhage has been neglected for decades. This is more noticeable when compare search trends (Figure 1) and number of publications with the ischemic stroke counterparts.1
Legend: This figure derived from Google search trends (Mesh terms: ischemic stroke and intracerebral hemorrhage) represent search interest relative to the highest point on the chart for the given time from June 2006 to June 2023 (17 years). A value of 100 is the peak popularity for the term. A value of 50 means that the term is half as popular. A score of 0 means there was not enough data for this term.
In the present study (Published in Lancet on May 25, 2023),2 the authors evaluated the implementation of a care bundle (vs. usual care) among eligible patients with an intracerebral hemorrhage (ICH) from 9 low-middle income and 1 high-income country. The intervention consisted in four measures: intensive BP reduction <140 mm Hg), strict glucose control (target 6·1–7·8 mmol/L in those without diabetes and 7·8–10·0 mmol/L in those with diabetes), antipyrexia treatment (target body temperature ≤37·5°C), and rapid reversal of warfarin-related anticoagulation (target international normalised ratio <1·5) within 1 h of treatment. Overall, 3221 patients with ICH assigned to the care bundle group and 3815 to the usual care group.
There was a lower likelihood of a poor functional outcome among participants in the care bundle group (common odds ratio 0.86; 95% CI 0.76–0.97; p=0.015). The favourable shift in mRS scores in the care bundle group was observed across a range of sensitivity analyses. There was also a reduction in death at 6 months (OR 0·77; 95%CI 0.63 to 0.95; p=0,015).2
Comments:
The authors and healthcare providers involved in the INTERACT 3 study are to be commended for their commitment of complying with the care bundle during the study period (May 27, 2017, and July 8, 2021).
INTERACT 3 Executive Members faced multiple challenges during the study that deserve comment:
First, the study was carried one during Covid-19 waves, which modified the paradigms of patient care worldwide.3,4
Second, the blood pressure (BP) variability between the care bundle vs. usual care groups were very modest as the adjusted mean difference at 24 h was -3·6 mm Hg (95% CI -4.5 to -2.7; p<0·0001). For example, readers can appreciate how close were BP trends between groups within 7 days in Fig 2 of the Lancet publication.2
There was a pronounced reduction in the mean systolic blood pressures from baseline in both groups (from 174.6 mmHg (SD 28·2) to 136.1 mmHg (SD 16.5) at 24 h in the care bundle group vs. 174.5 (SD 28.4) to 139.0 mm Hg (SD 17.2) at 24 h in the usual care group.
Readers may wonder how such modest differences in BP between groups could have accounted for the favorable outcomes, especially considering that over 90% of participants had an abnormal baseline BP (compared to smaller percentages of patients requiring active treatment for elevated glucose [34-37%], required antipyretic treatment or reversion of anticoagulation [<2%].2 Certainly, absolute BP reduction were more impressive within groups (-38.5 mmHg in the care bundle group and -35.5 mmHg in the usual care group). However, this observation also showed a modest average -3.5 mmHg reduction difference between groups.
NB: For readers concerned about worsening peri-hematoma ischemia (and clinical outcomes) by drastic reductions in BP, restricted diffusion lesions were observed among patients with over 60 mmHg systolic BP reduction for the management of ICH.5
Third, researchers involved in conducting clinical trials would appreciate the challenges of distinguishing a real effect from ‘noise’ related to biological small treatment effects between the active and control groups, probability of random unbalances between groups, the difficulties and variability related to the implementation of a strict protocol in an heterogenous research sample (low, middle and high impact countries).6
What was known and what we learned:
The proposed BP reduction for the management of ICH is not new. Previous trials showed the benefits of BP reduction <160 mmHg among patients with ICH.3,7-9 Most common predictors of poor outcomes in ICH, included: age, Glasgow Coma Scale, ICH volume and time from onset. The conceptualization that elevated BP increased the risk of hematoma expansion or poorer outcomes was already known from over 15 years ago.10 What was not knew is that a behavioral intervention – meaning the implementation, adherence, and delivery of a care bundle primarily targeting nurses and health care providers- could result in better clinical outcomes in the acute/subacute management of ICH. Changing behavior is a very difficult task, especially with limited incentives or when trying previously used (BP reduction) strategies.3,9,10 Furthermore, the benefits in clinical outcomes of the care bundle implemented in INTERACT 3 are not early perceived by health care providers during the intervention. In other words, it is admirable their commitment to adhere to a protocol hoping for benefits, but not witnessing immediate effects or rewards.
In conclusion, INTERACT 3 is a pragmatic study showing that the prompt and efficacious implementation of a low-cost care bundle was associated with a reduction in the likelihood of poor clinical outcomes after an ICH. We should underline key words “prompt’ (< 1 hour) and ‘efficacious’ as critical components of the proposed recipe. This is an intervention that requires no technology. A simple stethoscope, a sphygmomanometer (or BP machine), a glucometer, a thermometer, and INR values would suffice. Favorable results can be achieved in at least 90% of individuals with the first elements. These results bring hope for those living in areas with limited resources who are devoted to provide care with compassion and expecting to achieve better clinical outcomes for their ICH patients.
Paraphrasing Goethe,…”Willing is not enough; we must do”. INTERACT 3 investigators did it. Bravo !
References
- Broderick JP, Grotta JC, Naidech AM, Steiner T, Sprigg N, Toyoda K, Dowlatshahi D, Demchuk AM, Selim M, Mocco J, et al. The Story of Intracerebral Hemorrhage. Stroke. 2021;52:1905-1914. doi: doi:10.1161/STROKEAHA.121.033484
- Ma L, Hu X, Song L, Chen X, Ouyang M, Billot L, Li Q, Malavera A, Li X, Munoz-Venturelli P, et al. The third Intensive Care Bundle with Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT3): an international, stepped wedge cluster randomised controlled trial. Lancet. 2023. doi: 10.1016/S0140-6736(23)00806-1
- Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, Lindley R, Robinson T, Lavados P, Neal B. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl j Med. 2013;368:2355-2365.
- Collings DG, McMackin J, Nyberg AJ, Wright PM. Strategic human resource management and COVID‐19: Emerging challenges and research opportunities. Journal of Management Studies. 2021;58:1378.
- Garg RK, Liebling SM, Maas MB, Nemeth AJ, Russell EJ, Naidech AM. Blood Pressure Reduction, Decreased Diffusion on MRI, and Outcomes After Intracerebral Hemorrhage. Stroke. 2012;43:67-71. doi: doi:10.1161/STROKEAHA.111.629493
- Tonning ML, Kessing LV, Bardram JE, Faurholt-Jepsen M. Methodological Challenges in Randomized Controlled Trials on Smartphone-Based Treatment in Psychiatry: Systematic Review. J Med Internet Res. 2019;21:e15362. doi: 10.2196/15362
- Qureshi AI, Huang W, Lobanova I, Barsan WG, Hanley DF, Hsu CY, Lin C-L, Silbergleit R, Steiner T, Suarez JI. Outcomes of intensive systolic blood pressure reduction in patients with intracerebral hemorrhage and excessively high initial systolic blood pressure: post hoc analysis of a randomized clinical trial. JAMA neurology. 2020;77:1355-1365.
- Li Q, Warren AD, Qureshi AI, Morotti A, Falcone GJ, Sheth KN, Shoamanesh A, Dowlatshahi D, Viswanathan A, Goldstein JN. Ultra‐early blood pressure reduction attenuates hematoma growth and improves outcome in intracerebral hemorrhage. Annals of Neurology. 2020;88:388-395.
- Manning L, Hirakawa Y, Arima H, Wang X, Chalmers J, Wang J, Lindley R, Heeley E, Delcourt C, Neal B, et al. Blood pressure variability and outcome after acute intracerebral haemorrhage: a post-hoc analysis of INTERACT2, a randomised controlled trial. Lancet Neurol. 2014;13:364-373. doi: 10.1016/s1474-4422(14)70018-3
- Anderson CS, Huang Y, Wang JG, Arima H, Neal B, Peng B, Heeley E, Skulina C, Parsons MW, Kim JS. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. The Lancet Neurology. 2008;7:391-399.
WRITTEN INTERVIEW TO PROF. CRAIG ANDERSON
1. What did you set out to study?
INTERACT3 was designed to determine the effectiveness of implementing a package of promising interventions, organised as a time-dependent, target-based, quality improvement, called a ‘Care Bundle’, as compared to usual standard of care, in hospitals involved in the management of patients with acute intracerebral hemorrhage (ICH) across mainly low- and middle income countries. The Care Bundle included early intensive blood pressure lowering (systolic target <140mmHg), glycemic control, antipyrexia treatment, and reversal of warfarin related anticoagulation, all of which were to occur to normalized treatment targets within 1 hour of initiation in all patients as part of routine care.
2. Why this topic?
ICH is the most serious type of stroke without a clearly proven treatment that is a major health burden in low- to middle-income countries where there is a high prevalence of hypertension and other risk factors. Combining several promising interventions together that could be readily applied into health systems could have synergistic effects as part of active management and organised care.
3. What were the key findings?
Compared to usual (control) standard of care, implementation of the Care Bundle significantly improved the likelihood of functional recovery, reduced mortality, improved health-related quality of life, and reduced time in hospital.
4. Why is it important? Or how might these results impact clinical practice?
This is the first clinical trial to show a significant beneficial effect on a primary outcome of functional recovery in patients with ICH. Our approach to use a stepped wedge, cluster randomised design shows for the first time that organised systems of care with an active protocolized management plan associated with managing abnormal physiological variables improves outcomes in ICH. The benefits were consistent across all types of patients, even those with severe ICH or who require neurosurgery intervention, which has not been considered possible before as medical treatments are not considered potent in these situations.
5. What surprised you most?
Individually, the separate components of the Care Bundle produced relatively small changes in physiological variables. So, what’s really key to the effectiveness is the whole package of the Care Bundle, where the interventions have synergistic effects within themselves but are also likely to produce flow-on effects in the management of patients that are not so easy to measure.
6. What’s next for this research?
While INTERACT3 was both a research discovery and implementation form of clinical research which should accelerate the uptake of the findings into clinical practice, there is always barriers to the implementation of evidence into practice which will need to be worked upon across the globe. While the Care Bundle may seem low-cost and simple to apply in high-income countries, there are sill issues to be addressed to ensure that many patients with ICH receive such proven therapy in low- and middle-income countries, as well as low-resource settings in high-income countries. There is also some uncertainty over the time window for the effectiveness of components of the Care Bundle, in particular for intensive blood pressure lowering. The ongoing INTERACT4 clinical trial being undertaken in over 2000 patients in China should shed light on whether it is effective to apply intensive blood pressure lowering in the pre-hospital ambulance setting. These results will be presented in 2024.
7. Is there anything you’d like to add?
The INTERACT3 trial was a mammoth study conducted over several years that included addressing challenges of the COVID pandemic as well as other obstacles. I am very grateful for the dedication and hard work from my research team but also all the many hundreds of investigators associated with the study for their commitment to the mission of this work, which has been a very rewarding experience.
VIDEO INTERVIEW