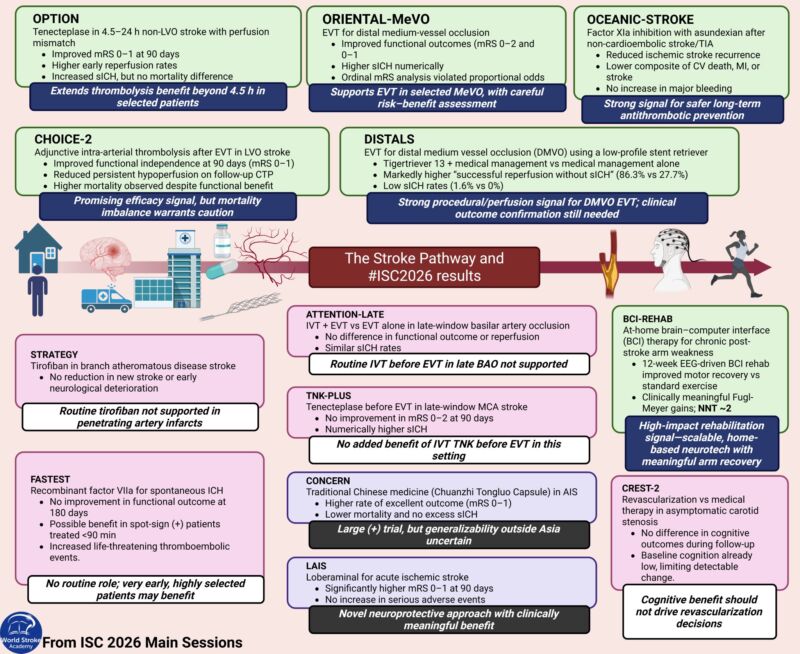
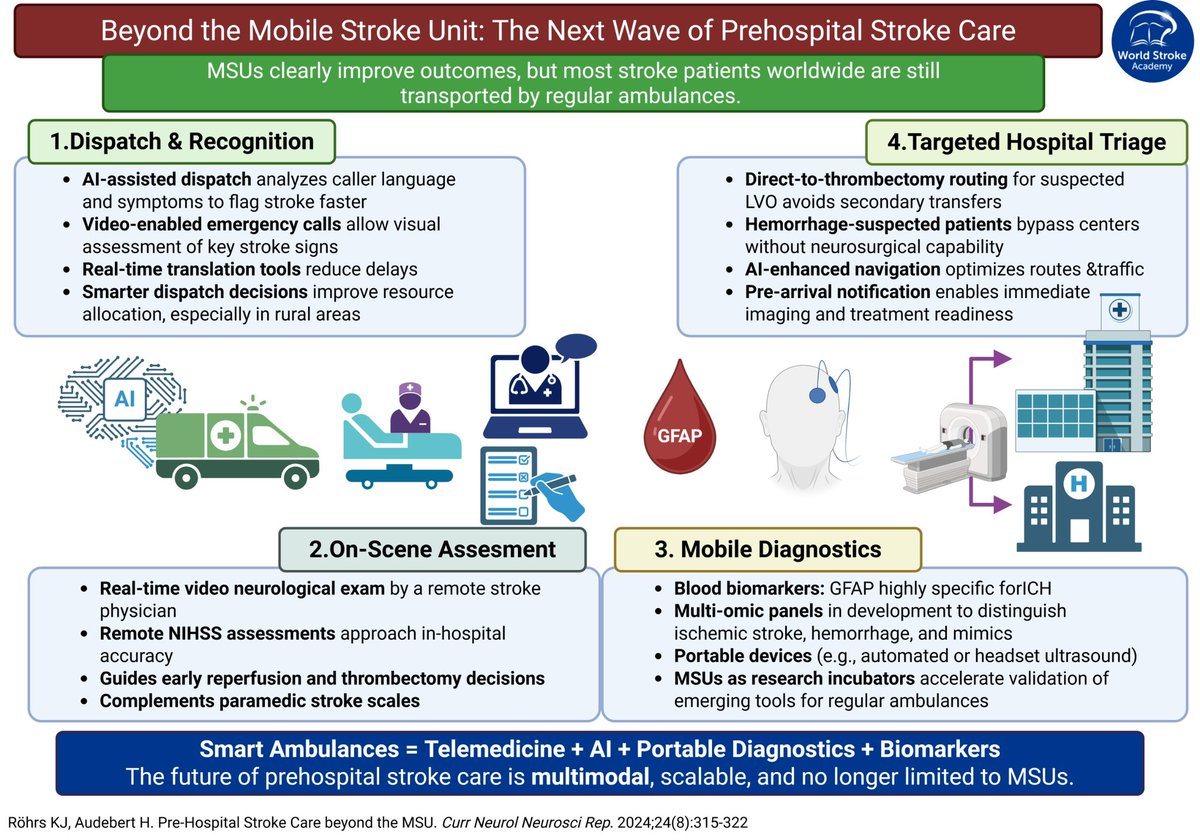
The Paper of The Month – November
21 Nov 2023Are antiplatelets safe and effective for secondary stroke prevention after intracerebral haemorrhage?
Title: Are antiplatelets safe and effective for secondary stroke prevention after intracerebral haemorrhage?
Author: Prof. Dr. Anita Arsovska – WSA Associate Commissioning Editor
This article is a commentary on the following:
Al-Shahi Salman R, Greenberg SM. Antiplatelet Agent Use After Stroke due to Intracerebral Hemorrhage. Stroke. 2023 Nov 2. doi: 10.1161/STROKEAHA.123.036886 (https://www.ahajournals.org/doi/10.1161/STROKEAHA.123.036886)
Summary of the findings:
This article provides an updated review about antiplatelets’ use to reduce the high risk of major adverse cardiovascular events (MACEs) after stroke due to spontaneous (nontraumatic) intracerebral hemorrhage (ICH) (1). It complements earlier updates about blood pressure-lowering, lipid-lowering, and oral anticoagulation or left atrial appendage occlusion for atrial fibrillation after ICH. When used for secondary prevention in people without ICH, antiplatelets reduce the risk of MACE by nearly 20% (rate ratio, 0.81 [95% CI, 0.75-0.87]), but might increase the risk of ICH (rate ratio, 1.67 [95% CI, 0.97-2.90]). Before 2019, guidance for clinical decisions about antiplatelet agent use after ICH has focused on estimating patients’ predicted absolute risks and severities of ischemic and hemorrhagic MACEs and applying the known effects of these drugs in people without ICH to estimate whether individual ICH survivors in clinical practice might be helped or harmed by antiplatelets. In 2019, the main results of the RESTART (Restart or Stop Antithrombotics Randomized Trial) randomized controlled trial including 537 survivors of ICH associated with antithrombotic drug use showed that antiplatelet agents might not increase the risk of recurrent ICH compared to antiplatelet agent avoidance over 2 years of follow-up (12/268 [4%] versus 23/268 [9%]; adjusted hazard ratio, 0.51 [95% CI , 0.25-1.03]; P=0.060). Guidelines in the United States, Canada, China, and the United Kingdom and Ireland have classified the level of evidence as B and indicated that antiplatelet agents may be considered/reasonable after ICH among patients with an increased CV risk. Three subsequent clinical trials have recruited another 174 participants with ICH, but they will not have power to determine the effects of antiplatelet therapy on all major adverse cardiovascular events reliably when pooled with RESTART.
ASPIRING (Antiplatelet Secondary Prevention International Randomized Study After Intracerebral Hemorrhage) aims to recruit 4148 ICH survivors to determine the effects of antiplatelet agents after ICH definitively overall and in subgroups.
Commentary:
Despite continuous research and clinical guidelines regarding the use of antiplatelets in secondary stroke prevention (ICH patients including), there are still some uncertainties among physicians regarding their appropriate use. For example, Forfang et al. (2023) assessed stroke physicians’ opinions about whether patients with previous ICH should receive antithrombotic treatment to prevent ischemic events and their views on randomizing patients in trials assessing this question (2). The results showed that in Scandinavia, 19 (21%) stroke physicians were uncertain about antiplatelet treatment after ICH for ischemic stroke or transient ischemic attack (TIA) and 21 (24%) for prior myocardial infarction. In the United Kingdom, 116 (77%) stroke physicians were uncertain for ischemic stroke or TIA and 115 (717%) for ischemic heart disease. In both regions combined, 191 of 250 (76%) would consider randomizing ICH survivors in a trial of starting versus avoiding antiplatelets. The authors concluded that considerable proportions of stroke physicians in Scandinavia and the United Kingdom were uncertain about antithrombotic treatment after ICH, so these findings support the need for such trials.
These uncertainties are most likely caused by the possible antiplatelets’ harmful effect by increasing the risk of bleeding.
Choi et al. (2023) aimed to analyze the risk and risk fractions for antithrombotics in sICH in South Korea because of increased concern about sICH with increased use of antithrombotic agents (3). From the National Health Insurance Service-National Sample Cohort including 1,108,369 citizens, 4,385 cases, aged 20 years or more and newly diagnosed as sICHs between 2003 and 2015, were included in this study. A total of 65,775 sICH-free controls were randomly selected at a ratio of 1:15 from individuals with the same birth year and sex according to a nested case-control study design. The results showed that although the incidence rate of sICHs started to decrease from 2007 onward, the use of antiplatelets, anticoagulants, and statins continued to increase. Antiplatelets (adjusted odds ratio [OR] 3.59, 95% confidence interval [CI] 3.18–4.05), anticoagulants (adjusted OR 7.46, 95% CI 4.92–11.32), and statins (adjusted OR 1.98, 95% CI 1.79–2.18) were significant risk factors for sICHs even after adjusting for hypertension, alcohol intake, and cigarette smoking. From 2003–2008 to 2009–2015, the population-attributable fractions changed from 28.0% to 31.3% for hypertension, from 2.0% to 3.2% for antiplatelets, and from 0.5% to 0.9% for anticoagulants. The authors concluded that antithrombotic agents are significant risk factors for sICHs, and their contribution is increasing over time in South Korea. These findings should draw the attention of clinicians to precautions to be taken when prescribing antithrombotic agents.
Reale et al. (2023) aimed to assess whether haemorrhagic transformation (HT) of acute ischemic stroke after reperfusion treatment and its severity influences the start of secondary prevention therapy and increases the risk of stroke recurrence (4). They performed a retrospective dual-center study, where they recruited ischemic stroke patients treated with thrombolysis, thrombectomy or both. Primary outcome was the time between revascularization and the start of any secondary prevention therapy. Secondary outcome was ischemic stroke recurrence within three months. They compared patients with vs. without HT and no (n = 653), minor (n = 158) and major (n = 51) HT patients using propensity score matching. The median delay in startingntithrombotics or anticoagulants was 24 h in no HT, 26 h in minor HT and 39 h in major HT. No HT and those with minor HT patients had similar rates of any stroke recurrence (3.4% (all ischemic) vs. 2.5% (1.6% ischemic plus 0.9% hemorrhagic)). Major HT patients had a higher stroke recurrence at 7.8% (3.9% ischemic, 3.9% hemorrhagic), but this difference did not reach significance. A total of 22% of major HT patients did not start any antithrombotic treatment during the three-month follow-up. The authors concluded that the presence of HT influences the timing of secondary prevention in ischemic stroke patients undergoing reperfusion treatments. Minor HT did not delay the start of antithrombotics or anticoagulants compared to no HT, with no significant difference in safety outcomes. Major HT patients remained a clinical challenge with both a delayed or lacking start of treatment. In this group, they did not see a higher rate of ischemic recurrence; however, this may have been censored by elevated early mortality. While not reaching statistical significance, hemorrhagic recurrence was somewhat more common in this group, warranting further study using larger datasets.
Nomura et al. (2023) aimed to investigate prior concomitant use of non-vitamin K antagonists and antiplatelets (5). They conducted an observational, multicenter, registry of 1043 patients with stroke (ischemic stroke, transient ischemic attack and TIA) receiving oral anticoagulants (OACs) in Japan (PASTA registry). Among the 216 patients with ICH, 118 (54.6%), 27 (12.5%), 55 (25.5%), 16 (7.4%) were taking NOAC monotherapy, NOAC and AP, VKA, and VKA and AP, respectively. In-hospital mortality rates were the highest in VKA and AP (31.3%) than in NOACs (11.9%), NOACs and AP (7.4%), and VKA (7.3%). Multivariate logistic regression analysis showed that concomitant use of VKA and AP (odds ratio [OR], 20.57; 95% confidence interval [CI], 1.75–241.75, p = 0.0162 ), initial National Institutes of Health Stroke Scale score (OR, 1.21; 95%CI, 1.10–1.37, p < 0.0001), hematoma volume (OR, 1.41; 95%CI, 1.10–1.90, p = 0.066), and systolic blood pressure (OR, 1.31; 95%CI, 1.00–1.75, p = 0.0422) were independently associated with in-hospital mortality. The authors concluded that although VKA in addition to AP therapy could increase the in-hospital mortality, NOAC and AP did not increase the hematoma volume, stroke severity, or mortality compared to NOAC monotherapy. However, these results should be interpreted with caution due to the small number of outcomes and small sample size that explain large 95% CI.
Asad and Kozberg (2023) review focused on ischemic stroke prevention in ICH survivors, particularly the use of antithrombotic medications and the management of hyperlipidemia and hypertension (6). They concluded that antiplatelets are generally safe in ICH survivors, but they should not be used without an indication. Shared decision-making is paramount including individualized risk stratification and exploration of potential alternatives to antithrombotic therapy among patients with ICH. Additionally, standardized imaging protocols (SWI, GRE sequences) and the use of biomarkers (plasma Aβ40)may inform the increased risk of recurrent ICH and inform treatment choices (7).
Conclusion:
We need more data and biomarkers that will identify patients with ICH with increased recurrent bleeding risk in order to optimize their secondary stroke prevention.
References:
1. Al-Shahi Salman R, Greenberg SM. Antiplatelet Agent Use After Stroke due to Intracerebral Hemorrhage. Stroke. 2023 Nov 2. doi: 10.1161/STROKEAHA.123.036886.
2. Forfang E, Larsen KT, Salman RA, Bell SM, Wester P, Berge E, Wyller TB, Rønning OM. Antithrombotic treatment after intracerebral hemorrhage: Surveys among stroke physicians in Scandinavia and the United Kingdom. Health Science Reports. 2023 Jan;6(1):e1059.
3. Choi HH, Jang D, Na H, Hong N, Lee SH, Kim KM, Kang HS, Kim JE, Shin A, Cho WS. Population-Attributable Risk Fractions for Antiplatelets and Anticoagulants in Spontaneous Intracranial Hemorrhages. Cerebrovascular Diseases. 2023 Mar 8:1-0.
4. Reale G, Caliandro P, Moreira TT, Almqvist H, Giovannini S, Grannas D, Kotopouli MI, Laurienzo A, Löfberg H, Moci M, Sköldblom S. Timing of Antithrombotic Secondary Prevention in Patients with Intracranial Hemorrhage after Stroke Thrombolysis and Thrombectomy. Journal of Clinical Medicine. 2023 Apr 7;12(8):2771.
5. Nomura K, Suda S, Abe A, Iguchi Y, Yagita Y, Kanzawa T, Okubo S, Fujimoto S, Kimura K, PASTA study group. Vitamin K antagonists but not non-vitamin K antagonists in addition on antiplatelet therapy should be associated with increase of hematoma volume and mortality in patients with intracerebral hemorrhage: A sub-analysis of PASTA registry study. Journal of the Neurological Sciences. 2023 May 15;448:120643.
6. Asad SD, Kozberg MG. Ischemic Stroke Prevention After Intracerebral Hemorrhage. Current Treatment Options in Cardiovascular Medicine. 2023 Jun 26:1-21.
7. Muir RT, Ismail Z, Black SE, Smith EE. Comparative methods for quantifying plasma biomarkers in Alzheimer’s disease: Implications for the next frontier in cerebral amyloid angiopathy diagnostics. Alzheimer’s & Dementia. 2023 Oct 31.